Biological drugs, protein-based products derived from living cells using biotechnology, have been used since the 1990’s as a treatment option for cancer patients. Since 2009, biosimilar therapies (therapeutically equivalent subsequent entry biologics) have been available in different therapeutic areas in Canada, and are now on the verge of becoming available for use in oncology. MD Analytics asked 31 Canadian Oncologists and Hematologists for their views on the introduction of biosimilar therapies for cancer treatment.
Read More
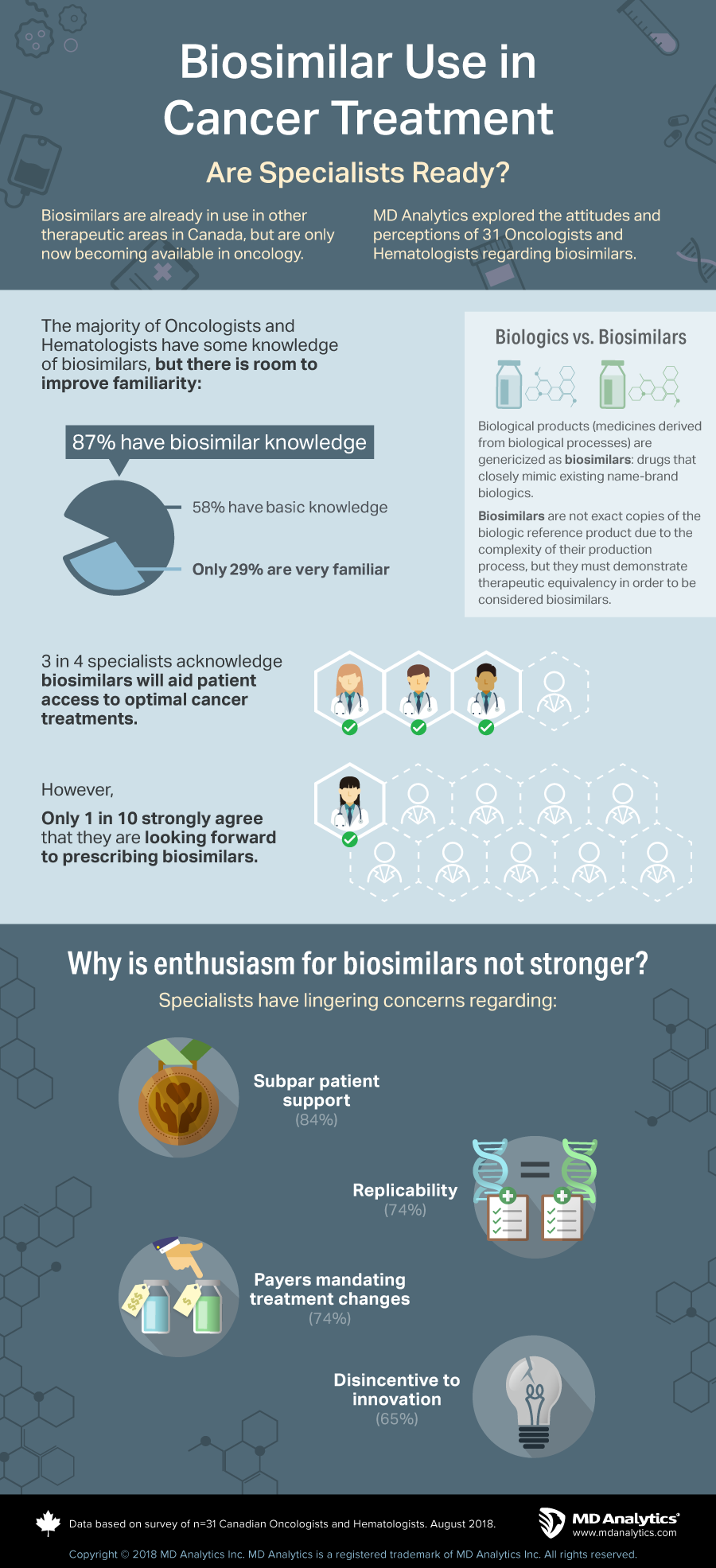
The majority of Oncologists and Hematologists have some knowledge of biosimilars, but there is room to improve familiarity. 87% have biosimilar knowledge; 58% have basic knowledge; only 29% are very familiar. 3 in 4 specialists acknowledge biosimilars will aid patient access to optimal cancer treatments. However, only 1 in 10 strongly agree that they are looking forward to prescribing biosimilars.
Why is enthusiasm for biosimilars not stronger? Specialists have lingering concerns regarding: subpar patient support (84%); replicability (74%); payers mandating treatment changes (74%); disincentive to innovation (65%).