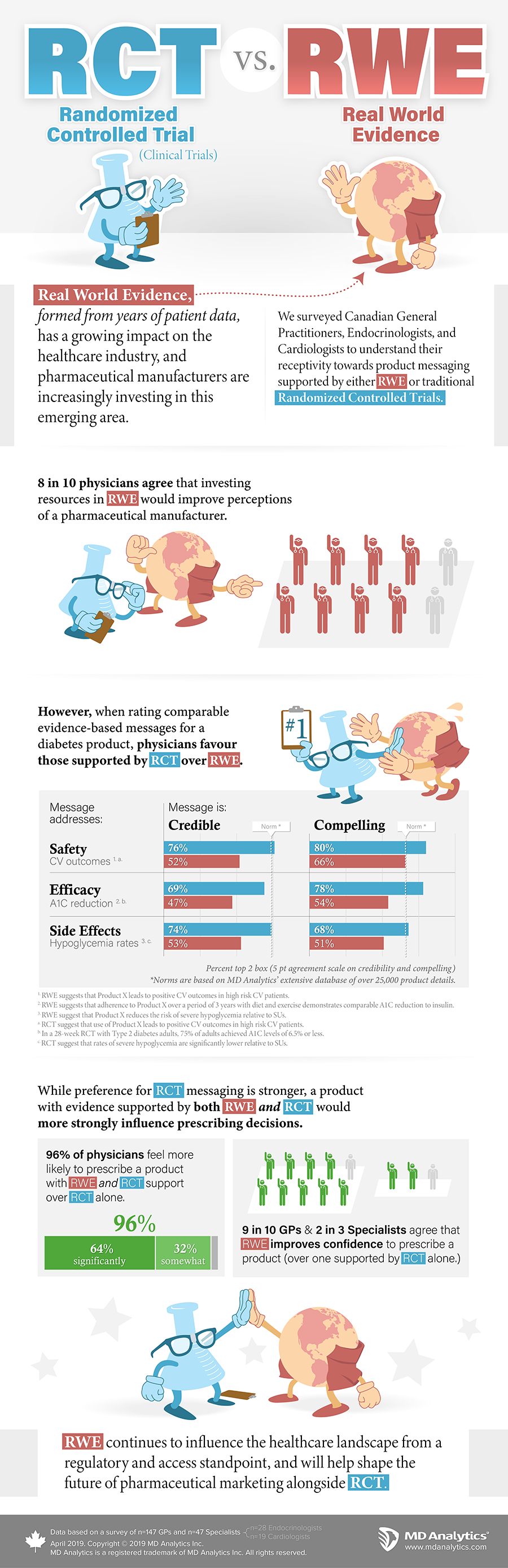
Real World Evidence (RWE) influences prescribing decisions and is a growing area of research that is based on a model driven by real world patient data. RWE stems primarily from an observational analysis of a sample in a less controlled environment. Focused on the efficacy and outcomes of therapies in a real world setting, RWE provides a unique lens not attainable from traditional clinical trial research. As a result, it may have a significant impact from a regulatory standpoint, and be a driving force in shifting the access landscape.
Read More
As Real World Evidence continues to shape the future of healthcare and medical treatments, pharma manufacturers want to better understand RWE’s impact on messaging strategies, specifically in comparison with randomized controlled trials. We surveyed physicians to better understand their perceptions on how product messaging derived from RWE are perceived compared to those produced through Randomized Controlled Trial (RCT) studies.
Based on data from 194 physicians practicing in Canada, 8 in 10 physicians agree that investing resources in RWE would improve perceptions of a pharmaceutical manufacturer. 96% of physicians are more likely to prescribe a product with RWE and RCT support over RCT alone. RWE continues to influence the healthcare landscape from a regulatory and access standpoint, and will help shape the future of pharmaceutical marketing alongside RCT.